✔ 100% Authentic Product
👁️ Currently Viewing 4132
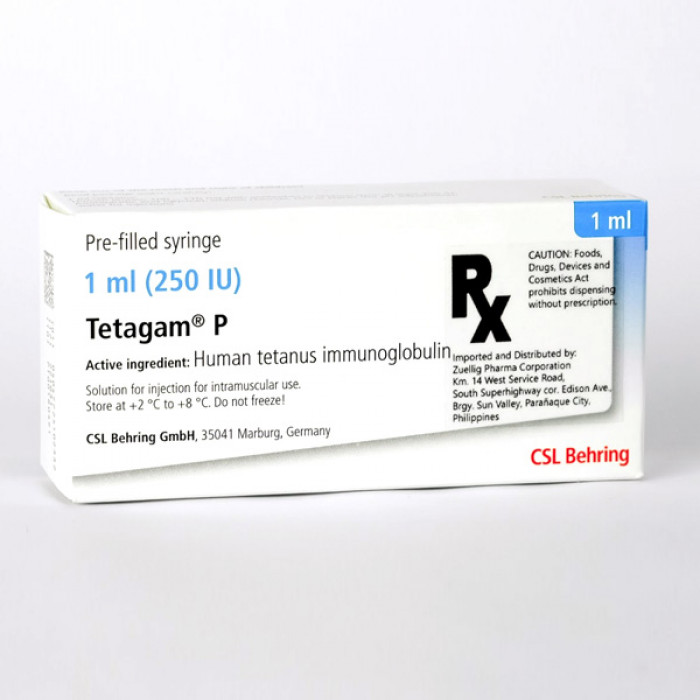
Tetagam-P 250 IU/ml Injection
Human Tetanus Immunoglobulin is used in the treatment and prevention of Tetanus.
Discount
Price: ৳ 1,287
MRP:
৳
1300
1%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
- Human Tetanus Immunoglobulin is a toxoid vaccine used to prevent tetanus, a serious bacterial infection that affects the nervous system.
- It contains Tetanus Immunoglobulin, which helps develop immunity by stimulating the body's immune system to produce antibodies against tetanus.
- Human Tetanus Immunoglobulin should be administered by a healthcare professional and should not be self-administered.
- Inform your doctor if you are allergic to Human Tetanus Immunoglobulin or any other vaccines.
- Disclose any history of seizures, fever, infection, bleeding disorders, low platelet levels, or weakened immune system due to HIV/AIDS.
- Inform your doctor about all prescription and non-prescription medications, vitamins, and herbal supplements you are taking.
- Consult your doctor before receiving Human Tetanus Immunoglobulin if you are pregnant, planning pregnancy, breastfeeding, or undergoing radiation or chemotherapy.
- Children need to receive all vaccination doses as recommended by healthcare professionals.
Safety Advices

Alcohol
UNSAFE
No interaction was found/established. Please consult your doctor before using Tetagam-P 250 IU/ml Injection.

Pregnancy
CONSULT YOUR DOCTOR
If you are pregnant, consult, and seek advice from your doctor before receiving Tetagam-P 250 IU/ml Injection.

Breastfeeding
CONSULT YOUR DOCTOR
Please tell your doctor, if you are a breastfeeding mother before receiving Tetagam-P 250 IU/ml Injection.

Driving
SAFE
There are no known effects of Tetagam-P 250 IU/ml Injection on your ability to drive or operate machinery.

Kidney
SAFE IF PRESCRIBED
If you have or have a history or evidence of any liver-related diseases, please consult the doctor before taking Tetagam-P 250 IU/ml Injection.

Liver
SAFE IF PRESCRIBED
If you have or have a history or evidence of any kidney-related diseases, please inform the doctor before taking Tetagam-P 250 IU/ml Injection.
✔️ Uses of Tetagam-P 250 IU/ml Injection
Tetagam-P 250 IU/ml is indicated for:
- Prophylaxis of tetanus following injury in patients with incomplete or uncertain immunization.
- Treatment of tetanus.
✔️ How does Tetagam-P 250 IU/ml Injection work?
It works by giving your body the antibodies it needs to protect it against tetanus infection.
✔️ Side Effects of Tetagam-P 250 IU/ml Injection
Injection-site reactions such as pain, soreness, and tenderness may occur. Other rare side effects include increased temperature, angioneurotic edema, nephrotic syndrome, and anaphylactic shock.
✔️ Quick Quggestions:
- Include magnesium-rich foods like avocados, flax seeds, corn, oatmeal, curd, and pumpkin to relax blocked jaw muscles and alleviate pain and inflammation.
- Incorporate high-protein foods such as milk, eggs, and meat into your daily diet to reduce the effectiveness of tetanus bacteria.
- Consume foods and drinks high in Omega-3 fatty acids like flaxseeds, walnuts, soybean oil, salmon, and tuna fish to alleviate pain, swelling, and inflammation.
- Perform jaw exercises regularly to improve the condition and minimize pain.
- Maintain a healthy diet and stay well-hydrated.
- Engage in regular exercise to improve overall health and combat pain.
- Use essential oils for massages to increase circulation and promote relaxation.
- Tetagam-P 250 IU/ml Injection is administered to prevent tetanus infection and should be given by a doctor or nurse in a hospital setting.
- Inform your doctor if you have any blood disorders before taking Tetagam-P 250 IU/ml Injection.
- Inform your doctor if you are pregnant, think you may be pregnant, or are breastfeeding before receiving the injection.
- Keep records of when you receive your booster to track when you are due for your next one.
✔️ Pharmacology
Tetagam-P 250 IU/ml prevents tetanus toxoid from damaging tissue by binding to it, interfering with its interaction with human tissue. This prevents the toxoid from invading the nervous system and causing symptoms such as painful muscle spasms and autonomic dysfunction. The Clostridium tetani bacterium is killed by antibiotic treatment or the host's immune system, and the immune globulin-bound toxoid is likely broken down by immune cells.
✔️ Dosage of Tetagam-P 250 IU/ml Injection
Adult Dose:
- Intramuscular Post-exposure prophylaxis of tetanus: Single dose of 250 IU, with a possible increase to 500 IU in certain cases:
- Infected wounds where appropriate surgical treatment cannot be achieved within 24 hours.
- Deep or contaminated wounds with tissue damage and reduced oxygen supply, foreign body injury (e.g., bites, stings, or shots).
- Burns, frostbite, tissue necrosis, septicaemic abortion.
- Adults weighing more than average.
- In case of extensive burns, a second injection of 250 IU human tetanus immunoglobulin may be administered after the exudative phase of the burn has subsided (approximately 36 hours after onset), along with 0.5ml of tetanus vaccine in a different extremity with a separate syringe and complete immunization schedule.
- Therapy of clinically manifest tetanus: Single doses of 3,000 to 6,000 IU in combination with other appropriate clinical procedures.
Child Dose: Same as adult dose.
✔️ Administration of Tetagam-P 250 IU/ml Injection
Tetagam-P 250 IU/ml should only be administered intramuscularly. It should not be given intravenously. Care should be taken to use separate syringes for each patient to prevent transmission of infectious diseases. The injection site should be compressed after administration. It should not be given subcutaneously except in cases of severe coagulation disorders where intramuscular injection is contraindicated.
✔️ Interaction
Tetagam-P 250 IU/ml may reduce the efficacy of live vaccines.
✔️ Contraindications
- Hypersensitivity to any of the components of the product.
- Known hypersensitivity to human immunoglobulins.
- Patients with bleeding disorders have a history of immunoglobulin A (IgA) deficiency or severe anaphylactic reactions to plasma products.
✔️ Pregnancy & Lactation
The safety of human tetanus immunoglobulin in pregnancy has not been established in controlled clinical trials. However, long-lasting clinical experience with immunoglobulins suggests that no harmful effects on the course of pregnancy, fetus, or neonate are expected.
✔️ Precaution & Warnings:
- Tetagam-P 250 IU/ml should not be administered intravenously.
- Caution is advised in patients with systemic allergic reactions to immunoglobulin.
- Patients with severe thrombocytopenia or coagulation disorders should receive Tetagam-P 250 IU/ml only if the benefits outweigh the risks.
- Injection should be performed cautiously to ensure the needle is not in a blood vessel.
- Patients should be monitored for long periods after injection for any adverse effects.
✔️ Storage
Tetagam-P 250 IU/ml should be stored at +2°C to +8°C, protected from light, and kept out of reach of children. It should not be frozen, and any frozen product should be discarded.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.